FCC Requirements for Medical Body Area Networks
Published on April 2, 2019
Medical Body Area Networks: Rules and Regulations
Written By: Bob Buckiewicz
Bob is the Director of Hardware Development at LS Research. He has over 30 years of experience in the development of low power wireless communication systems. Bob is a graduate of the University of Illinois (BSEE 1980) holds four patents, has two patents pending, and is a winner of Electronic Design News (EDN) magazine's "Excellence in Design" award for development of the world's first frequency synthesized walkman portable radio.
What are the FCC rules and requirements for MBAN devices?
The Federal Communications Commission (FCC) regulatory requirements are generally present in Subpart E of Part 95, which sets forth technical requirements that apply across all Personal Radio Services. Although the rules have not yet been formally revised, the FCC has indicated that they will apply the existing MedRadio provisions in Part 95 to MBAN operations, modified as necessary to refer to MBAN devices and their associated frequency bands.

Figure 1 - MBAN Application Examples (courtesy Fujitsu Wireless)
What are the technical requirements for MBAN Devices?
MBAN devices must comply with the relevant FCC technical requirements related to permissible bandwidth, radiated power, transmitter operation, unwanted emissions, frequency stability and RF safety.
Maximum Transmitter Power
For transmitters operating in the 2360-2390 MHz band, the maximum EIRP shall not exceed the lesser of 1 mW or 10*log (B) dBm, where B is the 20 dB emission bandwidth in MHz. For transmitters operating in the 2390-2400 MHz band, the maximum EIRP shall not exceed the lesser of 20 mW or 16+10*log (B) dBm, where B is the 20 dB emission bandwidth in MHz.
Antenna Requirement
The antenna associated with any MedRadio transmitter must be supplied with the transmitter and shall be considered part of the transmitter subject to equipment authorization. Except for the 2390-2400 MHz band, no antenna for a MedRadio transmitter shall be configured for permanent outdoor use. In addition, any MedRadio antenna used outdoors shall not be affixed to any structure for which the height to the tip of the antenna will exceed three (3) meters (9.8 feet) above ground.
Transmitter Operation
The transmitter must cease operating in the 2360-2390 MHz band if it does not receive a control message permitting such operation such as when the device is out of range or operated outdoors. The shutdown process shall commence within 45 mS after loss of the communication link or receipt of the shutdown command form the MedRadio programmer transmitter. Transmissions may be redirected from the 2360-2390 MHz band to the 2390-2400 MHz band by use of a control message.
Authorized Bandwidth
An aggregate channel bandwidth of up to 5 MHz – consistent with existing MedRadio rules.
Frequency Stability
+/- 100 ppm over 0°C to 55°. The temperature range was expanded from the MedRadio requirement to allow for limited outdoor operation.
Frequency Monitoring
The FCC feels it is unnecessary to specify protocols to ensure spectrum sharing among MBAN systems and does not wish to adopt detailed procedures that might inadvertently inhibit the development of innovative methods that would allow more intensive use of the spectrum. Although note required, it is expected that contention-based protocols, including listen-before-talk (LBT), frequency monitoring, time slot synchronization and frequency hopping may be utilized.
The FCC expects that MBAN manufacturers will determine the appropriate level of communications reliability through the risk management activities involved with medical device design that is subject to oversight by the Food and Drug Administration (FDA). MBAN manufacturers should be given the flexibility to meet that level of communications reliability through whatever means they find appropriate. Because of frequency coordination for MBAN and AMT sharing, the FCC feels it is not necessary to adopt frequency monitoring rules to promote spectrum sharing between these services.
Duty Cycle Requirement
The FCC has decided not to adopt specific duty cycle limits, which typically allow the implementation of a contention-based protocol for achieving reliable MBAN system performance although the FCC indicates that manufacturers are likely to employ duty cycles.
Authorized Locations
MedRadio operation is authorized anywhere CB station operation is authorized except that use of Medical Body Area Network devices in the 2360-2390 MHz band is restricted to indoor operation within a health care facility registered with the MBAN coordinator. A health care facility includes hospitals and other establishments that offer services, facilities and beds for use beyond a 24 hour period in rendering medical treatment, and institutions and organizations regularly engaged in providing medical services through clinics, public health facilities, and similar establishments, including government entities and agencies such as Veterans Administration hospitals.
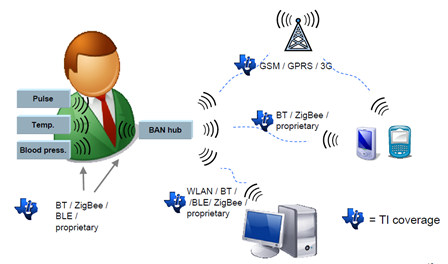
Figure 2 - Wireless Connectivity Solutions (courtesy TI & Arrow Medical Solutions)
Permissible Communications: Medical body-worn transmitters may only relay information in the 2360-2400 MHz band to a MedRadio programmer/control transmitter that is part of the same Medical Body Area Network (MBAN). A MedRadio programmer/control transmitter may not be used to relay information in the 2360-2400 MHz band to another MedRadio programmer/controller transmitter. Wireless retransmission of information to a receiver that is not part of the same MBAN shall be performed using other radio services that operate in spectrum outside of the 2360-2400 MHz band.
Frequency Registration and Coordination: The frequency registration and coordination are two separate but related processes. A health care facility that intends to operate a MBAN in the 2360-2390 MHz band must register the MBAN with a frequency coordinator (“the MBAN coordinator”) that the FCC will designate. The registration requirement will ensure that the locations of all MBAN operations in the 2360-2390 MHz band are recorded in a database. As part of the coordination process, the MBAN coordinator will first determine if a proposed MBAN in the 2360-2390 MHz band will be within line-of- sight of an AMT receiver. If the MBAN transmitter is within line-of-sight of an AMT receiver site, the MBAN and AMT coordinators will work cooperatively to assess the risk of interference between the two operations and determine the measures that may be needed to mitigate interference risk. The MBAN coordinator will notify the health care facility when coordination is complete and the MBAN must operate consistent with the terms of any agreement reached by the coordinators. If no agreement is reached, the MBAN will not be permitted to operate in the band
Interoperability: The FCC does not specify rules requiring interoperability although GE HealthCare supports a standard for interoperability (IEEE802.15.6) but it is voluntary.
- IEEE 802.15.6 Task Group 6 (BAN) is developing a communication standard optimized for low power devices and operation on, in or around the human body (but not limited to humans) to serve a variety of applications including medical, consumer electronics / personal entertainment and others.
- IEEE 802.15.4 WPAN Task Group 4j (TG4j) is working on an amendment to 802.15.4 which defines the physical layer for IEEE 802.15.4 in the 2360 to 2400 MHz band and complies with FCC MBAN rules. This amendment may also define modifications to the MAC needed to support this new physical layer.
MBAN Rules and Regulatory Updates in 2014 and beyond
With regulatory standards in place and a frequency coordinator to be selected shortly, semiconductor manufacturers can be expected to release MBAN compatible transceiver chipsets sometime in 2013. Based upon the regulatory requirements both a direct sequence spread spectrum (DSSS) similar to IEEE 802.15.4/ZigBee and a Frequency Hopping Spread Spectrum (FHSS) appear viable platforms that would allow multiple MBAN sensors to coexist within the same network. Because the FCC felt it unnecessary to specify protocols or other methods that would assure robustness and interoperability, each developer will need to determine their own level of communications reliability. This will need to occur at the system protocol level to allow coexistence of many MBANs devices within a hospital and at the hardware level, and to provide a degree of robustness to and from other high power wireless devices within the hospital, especially Wi-Fi devices that use the adjacent 2400-2483.5 MHz spectrum. It is also expected that the FCC will initially adopt a “permit but ask” policy that is typically associated with new technology, where devices are required to be tested and approved directly by the FCC test lab. In addition to the FCC approval, MBAN devices will also need to receive FDA approval before they can be used in hospitals.
Link to FCC Report and Order for MBAN
 Laird Connectivity is now Ezurio
Laird Connectivity is now Ezurio