The Risks of Using Unsecure Infusion Pumps
Published on August 5, 2015

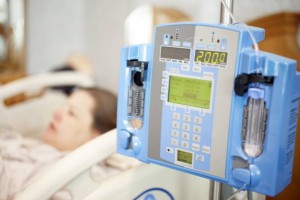
Infusion pumps are necessary when it comes to administering fluids such as medication and nutrients into a patient’s cardiovascular system. They serve the purpose of automatically distributing fluids during a patient’s stay which not only improves patient care, but helps to properly manage time, money, and resources. If infusion pumps are not managed correctly, they become impractical and unreliable for nursing staff. With enough stress on their shoulders, nurses need infusion pumps to run as seamlessly as possible, so they can focus on the most important aspect of their careers – patients.
Security amongst wireless medical devices is a crucial factor in providing quality care for patients. Not only does it protect devices against malfunction, but also from hackers accessing hospital networks and performing unauthorized actions such as changing drug doses of patients. On July 31, 2015, the FDA issued a safety communication urging hospitals to stop the use of Hospira Symbiq Infusion Systems due to the wireless devices being vulnerable to hacking.
Fortunately, the FDA and Hospira did not detect any unwarranted access of the Symbiq Infusion System. More importantly, no patients were harmed . However, the FDA is strongly encouraging hospitals and other healthcare facilities to transition to alternative infusion systems to prevent harm to patients and problems of wireless devices. Symbiq Infusion Systems affected are Versions 3.13 and prior.
The FDA stated, “Hospira has discontinued the manufacture and distribution of the Symbiq Infusion System, due to unrelated issues, and is working with customers to transition to alternative systems. However, due to recent cybersecurity concerns, the FDA strongly encourages health care facilities to begin transitioning to alternative infusion systems as soon as possible.”
The FDA recommended that healthcare providers disconnect affected products from their networks as soon as possible and warned that the disconnection process may have operational impacts. In order to prevent issues, administrators were urged to update drug libraries manually, but warned that manual updates can create a labor burden, as well as entry errors.
According to Qmed, Hospira reported that nearly 55,000 LifeCare PCA3 and PCA5 infusion pumps were being used throughout the world, while over 400,00 drug pumps were located in hospitals worldwide in May 2014. These large numbers caused a major inconvenience for healthcare providers, but also assured administrators that wireless medical security is a key factor in providing patients with the best possible care.
Laird is focused on providing customers with secure wireless devices from all aspects of the medical industry. To learn more about security in hospitals and how healthcare providers can choose devices that offer top security, visit Laird’s Connected Hospital webpage. Also, for technical insight on this topic, check out our white paper, “Testing Wi-Fi Functionality in Medical Devices”.
 Laird Connectivity is now Ezurio
Laird Connectivity is now Ezurio